EXTERNAL LINK
You are about to leave Pajunk.com. This link is provided strictly for information sharing purposes.
Pajunk GmbH assumes no responsibility for the quality, content, nature, or reliability of any linked site.
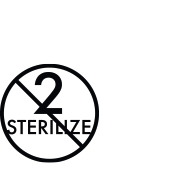
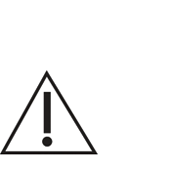
PAJUNK’s labelling is designed to meet all applicable international standards and regulations. Where possible, PAJUNK adopted the use of symbols to communicate requirements, product characteristics and provide guidance on handling and storage to the user. A compiled listing of symbols that may appear on the product labelling and the meaning of the symbol is provided in this document.